& Construction

Integrated BIM tools, including Revit, AutoCAD, and Civil 3D
& Manufacturing

Professional CAD/CAM tools built on Inventor and AutoCAD
9 min read
There’s energy behind everything in this world, from the juice in the batteries that keep your Xbox controller running to the force in the smack of a wooden bat that sends a ball flying out of the park. But not all energy is equal, and there’s one type in particular that has shaped our world of electronics more than any other – electromagnetic (EM) energy.
This power, which comes in the form of electromagnetic waves defies physical barriers, hurtling through the vacuum of space and opening up a world of discoveries in our modern times, from radio to radar, satellites, and more! To ever fully understand how wireless works in today’s electronics, you’ll need to take a trip to a ball game, d see how electromagnetic waves work in motion.
We’re bombarded by waves of various types at all hours of the day, each coming in different forms and flavors. For example, the crack of a baseball bat against a ball produces a sound wave which travels through a physical medium to get to your ears. And when everyone in the crowd stands up to do the wave and cheers, that’s sound waves in motion again. These sound waves, which get lumped into a category of mechanical waves, all require a physical object or medium to pass through to be heard.
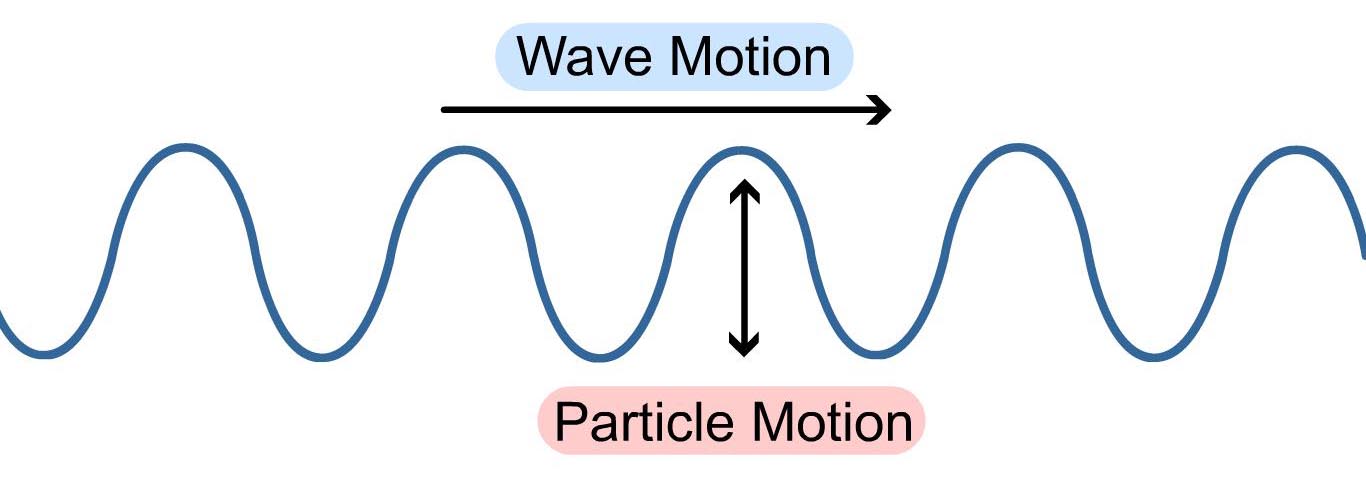
Unlike mechanical waves, electromagnetic waves don’t require the presence of physical medium, and you’ll find them hurtling through the emptiness of space without a second thought. Electromagnetic waves are unique in their composition, bringing together both electric and magnetic fields, which dance together in a perfect spiral as they go traveling about space as a transverse wave.
Because electromagnetic waves don’t need a physical medium to travel through to get from point A to B, they’re also the fastest wave known to man and can travel through a vacuum of space at the speed of 3.00 x 108 m/s! That’s not to say that these waves can’t travel through a physical medium, it just works a little differently when they do. Let’s break it down:
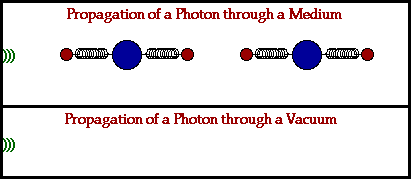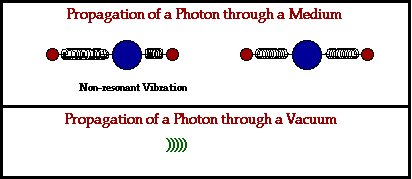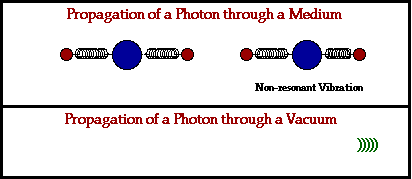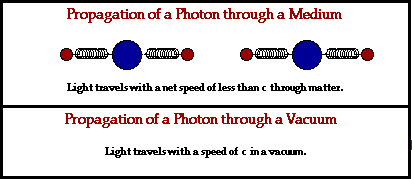
In a physical medium, this process of an electromagnetic wave getting absorbed and ejected from atom to atom will cause the wave to travel slightly slower than it does through a vacuum. The denser the physical material, the more delayed the electromagnetic wave will move.
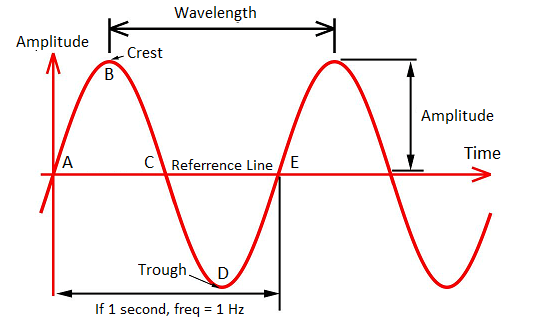
Before diving into all the forms of electromagnetic waves, first, we need to understand how these waves are measured, which also gives you a clue as to how they get organized in the spectrum. While all waves take different shapes, every electromagnetic wave that you’ll encounter has the same S-shaped (sine wave) curve as shown below. These are called transverse waves. You can measure these transverse waves in several ways:
Make Sense? Now we can get back to our electromagnetic spectrum. All electromagnetic waves are organized in a very detailed hierarchy, all based on our measurements of both frequency and wavelength. Electromagnetic waves on this spectrum progress in order of increasing frequency and decreasing wavelength, like so:
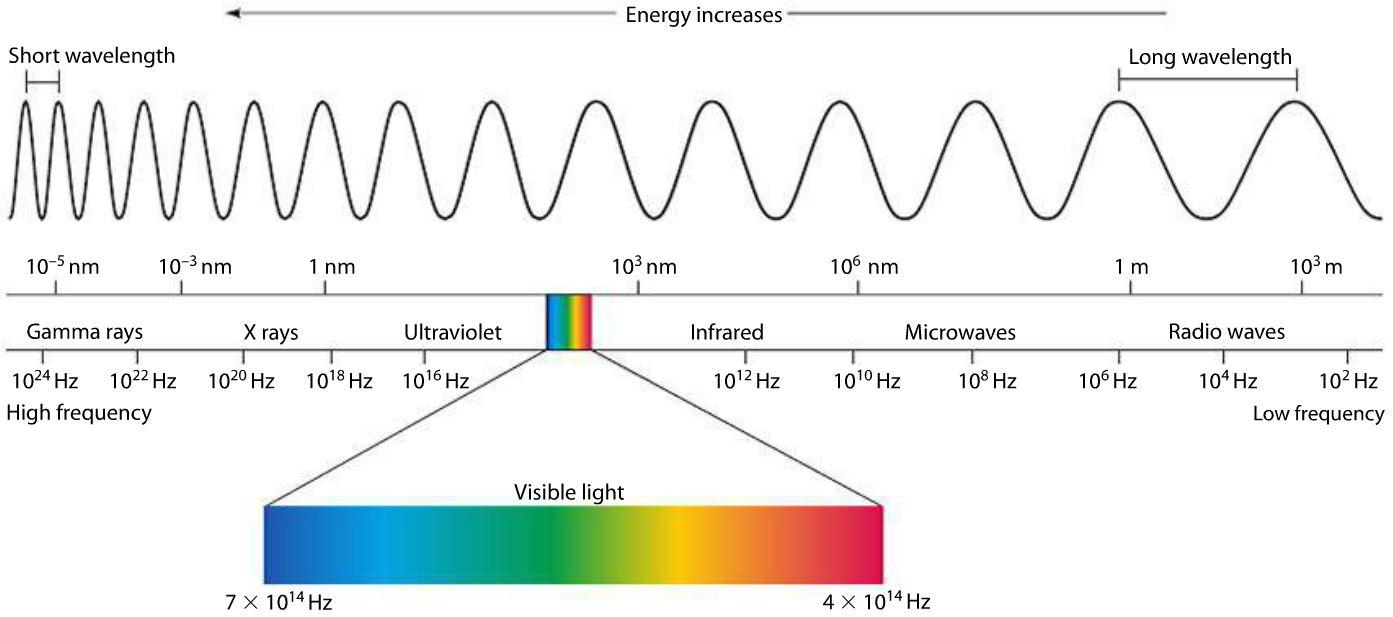
At the minimum end of the electromagnetic spectrum are radio waves, which have frequencies ranging from 30 gigahertz (GHz) to 3 kilohertz (kHz). As the name suggests, radio waves are most famous for their use in radio stations, and if you’re listening to AM radio, then you’ll be dialing into a specific radio frequency between 520 and 16010. AM radio stations are measured as thousands of hertz per second, called kilohertz (kHz).
You also have FM radio frequencies, which can be dialed in between 87.0 and 107.9 million hertz per second, called megahertz (MHz). Outside of traditional radio, you’ll also find radio waves powering nearly all of our wireless electronic systems, like WiFi, Bluetooth, cell phone signals, and even radar. Radio waves can even measure how fast a pitcher throws a baseball using a speed gun or speed camera!

Microwaves rest smack dab in the middle of radio waves and infrared waves and have a frequency between 3 gigahertz (GHz) and 30 terahertz (THz). You won’t find microwaves being used just to heat up your leftover for lunch though. Microwaves also have some traditional uses in other high-bandwidth devices, like radar, television, and satellites.
Before electromagnetic waves start to become visible, they take on the form of infrared waves. These have a frequency from 30 terahertz (THz) to 400 THz with wavelengths that measure as small as 0.00003 inches! Like all other waves before the visible spectrum, infrared is completely invisible to the human eye although they can be felt as heat.
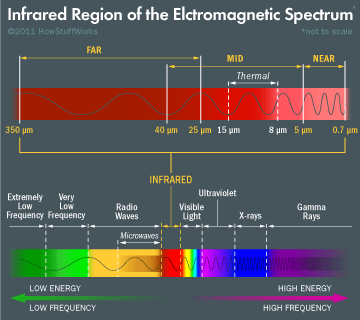
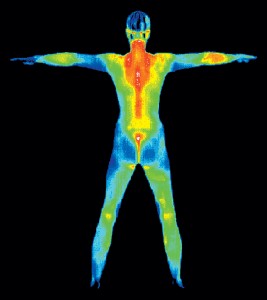
You’ll find infrared being used in TV remote controls, and also for thermal imaging for use in night vision goggles in all of your favorite spy movies. Your body also produces infrared waves, just like the sun!
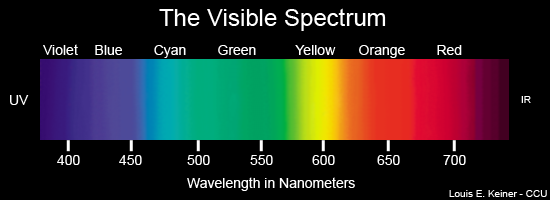
Finally, we come to the one and only visible part of the electromagnetic wave spectrum that our human eyes can behold visible light! This form of electromagnetic energy is visible to all of us as the spectrum of colors found in a rainbow. Colors have a particular wavelength on the electromagnetic spectrum, here’s just a few:
Beyond the visible light spectrum, we get into ultraviolet waves, which occur at high frequencies, sending more than 1,000 trillion cycles every second, with a wavelength measuring between 400 and 1 nanometers.
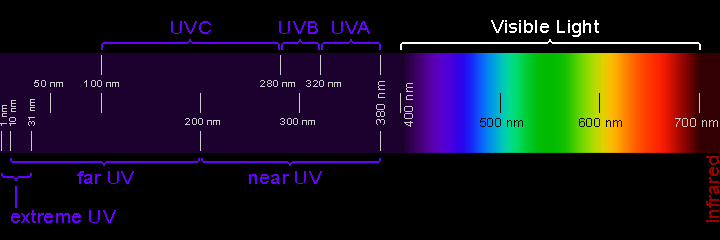
You’ll find UV waves being used to sterilize medical equipment and also used to fend off bacteria and viruses. You can also use UV waves to check for counterfeit money, which shows all of the hidden symbols that the US Federal Reserve prints on a legit dollar bill.

Next, we have the x-ray, and if you’ve ever broken a bone or been to the dentist, then you know exactly how this electromagnetic wave is used. The wavelengths in x-rays are so short that they’ll go flying past a given point at one million trillion wavelengths per second. At this point in the electromagnetic spectrum, you need to be careful with how much exposure you get to these waves. X-rays produce such an intense burst of energy that they can kill cells in your body if you contact them unprotected.
Gamma rays are the beasts of the electromagnetic spectrum, and wield enough power to break the bonds between molecules! Their frequencies are greater than 108 Hertz, with wavelengths measuring super tiny at only 100 picometers (that’s 4 x 10-9 in inches). As might be expected, gamma rays can cause some unpleasant damage to living tissue, which makes them perfect for attacking cancer cells. However, if you have an uncontrolled gamma ray exposure, like from a nuclear bomb, then you’re likely done-for.
Electromagnetic waves have many different varieties, and you might be wondering how we even came to discover such a mysterious and largely invisible force that powers our world. Our journey to discovery begins in the 1870s with Scottish scientist James Clerk Maxwell. Maxwell ended up putting together a theory when he saw that electrical and magnetic fields can couple together to form what we now know as electromagnetic waves. The relationship he discovered was called Maxwell’s Equations.
In 1888, German scientist Heinrich Hertz continued to expand on Maxwell’s observations, noticing that when he made an electrical spark jump between two terminals, a second flash would appear at the same time between another set of terminals yards away. This ability to manifest electromagnetic waves in their visible form led the introduction of Hertzian waves.

In 1896, things started to take off on the study of electromagnetic waves with Italian scientist Guglielmo Marconi. Marconi expanded upon Hertz’s original discovery to create the very first radio transmitter, which allowed him to send radio signals up to a mile away. These Hertzian waves that Marconi transmitted later became known as radio waves, which are still in use today.

Wireless technologies, and the electromagnetic waves that make them all possible are so full of mystery and wonder. By understanding their basic building blocks, you can in time play in the big leagues, wielding your own ability to send data flying across the room without a single wire! In our Wireless Electronics Basics series, electromagnetic waves will serve as the foundation for all of the impressive wireless technologies. Be sure to check back soon as we explore how WiFi, Bluetooth, RFID, NFC, and other wireless technologies work in more detail.
Ready to get started with your own wireless electronics project? Try Autodesk EAGLE for free today!
By clicking subscribe, I agree to receive the Fusion newsletter and acknowledge the Autodesk Privacy Statement.
Success!
May we collect and use your data?
Learn more about the Third Party Services we use and our Privacy Statement.May we collect and use your data to tailor your experience?
Explore the benefits of a customized experience by managing your privacy settings for this site or visit our Privacy Statement to learn more about your options.