Can Silicon Keep Delivering ‘Moore’?
A Look at the Future of Electronics with Graphene as King
Moore’s Law has been cruising at a steady click since 1965 with the manufacturing of the first integrated circuit, but it’s struggling to keep up these days. Just last year, Intel planned to introduce a 10nm processor, only to switch gears and stick with its tried-and-true 14nm until 2017. What’s going on here? Don’t get me wrong, it’s still a modern marvel that we can stuff up to 2 billion transistors into today’s consumer microprocessors, but it’s clear that our rapid pace of transistor expansion is coming to an end or at least slowing down.
And now the question on everyone’s mind is – can we still keep delivering ‘Moore’ of what we need with silicon alone?
Are the Days of Silicon Numbered?
You would not be reading this article on your computer or smartphone if it wasn’t for the legendary element silicon (Si). It is used in nearly every piece of electronics, powering the brains of the operation in our advanced microprocessors.

Pop open your computer, smartphone, or other electronic device and you’re bound to see an integrated circuit like this nestled inside. (Image source)
Since its use in the first microprocessor in 1965, silicon has been the reigning champ, packing in hundreds, millions, and now billions of transistors into the tiniest of spaces. Today, silicon is starting to show its weaknesses as the material of choice for the future of electronics. Why?
- Electrons Go Crazy. Many of today’s circuits are as small as 7nm wide, and when you’re trying to send electrons down transistor pathways in these tiny silicon spaces, they often become unstable and difficult to control. What do you do when electrons go rogue and start interfering with other signals? Hope for the best, I guess.
- Mobility Issues. There’s also the problem of electron mobility. Yeah, you can pack billions of transistors into a space the size of a red blood cell, but silicon itself doesn’t provide the best environment for electron mobility as other materials do, like indium or graphene.
- High Heat Problems. Another issue is that the more you pack into silicon, the higher the temperature climbs with all of that activity, leading to degraded performance. Today’s ICs with billions of transistors requires a ton of fans just to keep everything cool. Think of the giant heatsink strapped to your computer’s processor.
- Lazy With Light. Silicon is also terrible at transmitting light. And with a widespread use of lasers and LEDs, manufacturers are starting to use alternative semiconductor materials for photonic applications to work around their silicon deficiencies.
- Wasted Power. Despite all of the power pumping out of a silicon-based circuit, there’s also a ton of energy being lost in the process. Check out the graph below, and you’ll see what we mean. 20nm processors are already tipping the scales beyond 50/50 between usable power, and power that goes to waste.
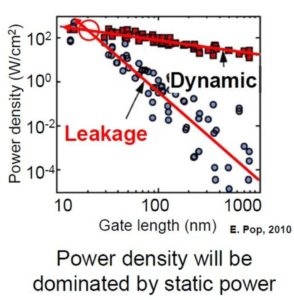
Read from right to left, this graph shows how wasted energy in our processors continues to decrease the smaller they get. (Graph from Carver Mead at the 2013 ISSCC)
Silicon has had a fantastic run, allowing us to transition from hundreds to billions of transistors in only a few decades. But perhaps it’s time to move beyond the industrial age of electronics and our almost obsessive focus on raw speed. We know what our electronics can do, but do they do what needs to be done efficiently? And how are we going to power all the devices in our Internet of Things (IoT) connected future? Bring on the graphene!
Graph What?
Remember back in your childhood when you had to get up in the middle of class, walk over to the pencil sharpener latched onto the wall, and hand crank away to transform your pencil into a useful instrument again? In the middle of that pencil of yours was graphite, the father of graphene. Slap together three million layers of graphene, and you’ll eventually have enough to make just one millimeter of your grade school pencil!

The essence of childhood right here, but can number 2 replace 7nm?(Image source)
The Carbon Family
Graphene is home to the carbon family, and it’s so thin, being only a single-atom height. This makes it as close as you’ll ever get to a 2D material living in a three-dimensional world. It can have plenty of length and width, but no perceivable height!
Looking under a microscope, you can quickly identify graphene by its hexagonal shape (like a honeycomb). This unique composition has four electrons in its outer shell, three of which join with other atoms to form the solid substance we can use. And the fourth electron? That’s where all the magic happens. Graphene has a ton of superpowers thanks to its four electrons, including:
- Super-Strength. Our little graphene superhero might be the thinnest material known to scientists, but it’s also 300 times stronger than steel and way harder than a diamond ever will be (diamonds are also part of the carbon family).
- Super-Flexibility. Ever wish you could sit on your smartphone without breaking it? If it was made with graphene, you could! This material is completely transparent and flexible and has some great potential for use in our consumer electronics.
- Super-Conductivity. Graphene is also one of the best conductors of both heat and electricity. This makes it the perfect alternative to both silicon and copper.
Check out the perfectly hexagonal shape of graphene, just like a honeycomb! (Image source)
And how was graphene discovered?
In 2004, two professors at the University of Manchester were able to extract graphene layer by layer using simple adhesive tape. They kept pulling off each layer one at a time, arriving finally at a layer that was a single layer of atoms. This great discovery kicked off a ton of research into the practical uses of graphene, and both Andre Geim and Konstantin Novoselov went on to receive Nobel prizes in 2010 for their discovery.

Here we have Konstantin (left) and Andre (right), discoverers of graphene via tape. (Image source)
Taking Off the Rose-Colored Glasses
Ok, let’s be real. Graphene is nowhere near ready for prime time. First, there’s still no way to reliably and cost-effectively manufacture this material. You’ll wind up paying over $800 just to get a usable gram of graphene made, and the only two proven methods for making it include:
Mechanical Exfoliation

Remember how our two scientists discovered graphene with the use of some tape? This approach is still in use, where layer by layer is peeled away from a chunk of graphite for experimental research.
Chemical Vapor Deposition

Another and more promising method is that of Chemical Vapor Deposition or CVD. In this process, chemical vapors get evaporated in a furnace, leaving behind deposits of graphene on a layer of metal. This process is also used to manufacture large integrated circuits, so it has the added benefit of a proven track record.
Outside of these two processes, there’s no other reliable or cost-effective method to manufacture graphene today, and graphene faces an uphill battle to be a viable alternative to silicon. Here are just a few of the questions on our mind:
- The Process. How much will manufacturers have to change their manufacturing process just to incorporate graphene? Will it require an entirely new set of certifications and standards?
- The Tools. Are manufacturers going to need brand new machines, or can they just retrofit old instruments to get the done job?
- The Practices. And most importantly, will manufacturers need new best practices to ensure the highest quality graphene production?
These are some tough questions to answer. Particularly for companies like Intel that can already pump out 50,000 microprocessors today with silicon without breaking a sweat. That transition time between silicon and graphene has to be done right, or not at all.
It’s Not a Semiconductor
Outside of the manufacturing realm, there’s also the very real issue that graphene isn’t a semiconductor, and doesn’t fit into the existing model of how transistors work. Yes, graphene is an excellent conductor, but how do you go about translating that into a working model that can operate as an integrated circuit?
While silicon is widely recognized for its ability to be modified via doping to carry an electrical charge, can that be replicated for graphene? Researchers have found that messing around with negative and positive charges in graphene-like you would in P-type, and N-type silicon can have some unintended consequences on graphene’s electrical properties.
Training a Workforce
And how do you go about training an entire engineering culture on how to work with graphene? Granted, this isn’t a new problem. When the first transistors rolled around, companies shoveled out the money and time to get their engineers up to speed, so it’s likely to happen again if graphene starts to take over
There’s also the issue of our design tools that we rely on for our daily work. We’ll likely need new simulation models and new ways of working with graphene in our layer stackups. And will printed circuit boards even be manufactured in the same way once graphene becomes commonplace? If you can use graphene in an integrated circuit, wouldn’t you start using it in other electronic components? This is already being done in capacitors, to create what are called supercapacitors (we’ll touch on this later). But how will these graphene-based additions change how we design, source and manufacture our components and boards?
The Many Awesome Uses for Graphene
Yes, graphene has a long, long way to go before it can become a viable alternative to silicon. But that still won’t stop us from dreaming about some of the amazing uses it can have for electronics and the world as a whole. Here’s our top three:
Unbreakable Touchscreens
The most immediate and obvious use for graphene would be to replace those flimsy touchscreens in our smartphones and tablets that are made from Indium, one of the rarest elements on Earth. With graphene’s superhero-like strength, 100% transparency, and yoga-like flexibility, we’d enter a whole new world of consumer electronics and hardware connectivity! And we’d also take advantage of a carbon-based material, which is one of the most abundant elements on Earth, unlike Indium.

A super flexible touchscreen OLED touchscreen produced by LG, could this be the future of our consumer devices? (Image source)
Supercharged Batteries
The use of graphene could also finally bring us into an age of powerful and lightweight batteries that would far exceed the capabilities of any battery in existence today. These supercapacitor batteries already being researched over at the University of California, Los Angeles and present some amazing possibilities. Check out the video below to see what we mean.
Imagine being able to plug your smartphone in, and having it charge in 30 seconds. And on an even bigger scale, what would happen if this kind of battery was available in electric vehicles? Could charging be as quick as a stop at the traditional gas station?
https://www.youtube.com/watch?v=-CP3zPHRv60
Cold Computers
You will never have to liquid cool your home-made PC anymore! Microprocessors in today’s computers can hit temperature upwards of 240°F, but with a layer of graphene applied to these same processors, temperatures dipped down to 55°F. That’s huge!
Imagine all of the energy that data centers alone can save by making microprocessors with graphene. Could this cut down on the enormous costs requires just to keep these data centers cool? Maybe we’ll find ourselves in a future where microprocessors and transistors work at lightning fast speeds, without the excess heat.

Today’s data centers pump out a ton of heat, but graphene can change that when used in microprocessors. (Image source)
That’s just a very small glimpse at all of the possibilities surrounding the use of graphene. There’s a ton of other applications available in a variety of industries, including:
- Solar. Using graphene in combination with the inorganic compound molybdenum disulfide produces a solar cell that’s 30x stronger than the thinnest solar cells around.
- Aerospace. Using graphene in airplanes would allow manufacturers to create super strong yet lightweight components that would lower the weight of planes and their fuel costs.
- Medical. Researchers at the British firm Oxford Nanopore are using graphene to speed up the processing of DNA sequencing. There’s also uses for graphene in creating ultra durable and flexible prosthetic limbs.
The New Electronics Material King?
It’s still too early to tell if graphene will dethrone silicon as the king of the electronics world, or the whole world for that matter. And while graphene has a ton of great uses that we can keep dreaming about, we still have to remember all of the hurdles to be overcome first. There’s the complexity of making it manufacturable, the enormous costs of training an engineering workforce, and all of the research required along the way. In short, all of this is going to take time, lots and lots of time. But don’t get so down. The first transistor came about in 1947, with the first IC showing up ten years later. It might take another decade or two to get graphene rolling, but at least the momentum has already started!
Until graphene arrives, you can still take advantage of the millions of and billions of transistors inside the free Integrated Circuit libraries in Autodesk EAGLE. Try Autodesk EAGLE for free today to get started!