Learn how lasers work to power our modern age of electronics and communications, including how they were invented, applications, and more.
The magic of lasers is all around us, from high-speed cutting machines to tattoo removal, eye surgery, and barcode scanners, the list can go on. This sometimes invisible technology is often perceived as a mystery even in our most cherished science fiction, with something like the Death Star using a super-laser to destroy entire planets. But what exactly is a laser, how does it work, and how do we use it to do some everyday amazing things? Like any other electronic technology, you might be surprised at how simple it all can be.
Lasers Defined
You can think of a laser as a machine that emits trillions of light particles, called photons, into a precise beam of light. The laser is an acronym that stands for light amplification by stimulated emission of radiation. The two key words there being light amplification, which is caused by a process of stimulated emission of light radiation. We’ll be covering this in more detail later.

At their core, lasers are not all that different from other technologies that use light on the electromagnetic spectrum. Whether you’re talking about radio waves, x-rays, infrared, or lasers, they’re all using parts of both the visible and invisible light spectrum to do their work. However, unlike other light technologies, lasers do have some unique characteristics, including:
- Monochromatic: The light emitted from a laser is a single wavelength of light, which is why you often see lasers as red or green. This wavelength and the resulting color are caused by the amount of energy released when an electron loses energy.
- Coherent: The light pattern from a laser is also coherent or organized. Take for example a flashlight, which emits a cone of photons with differing wavelengths in all directions. In a laser, all of the wavelengths in each photon line up perfectly with each other, like soldiers marching in a straight line.
- Directional: The light from a laser is directional. Compared with a flashlight which releases light in a variety of directions, lasers instead offer a precise and concentrated beam of electromagnetic radiation.
Three core components make every laser work, whether that’s a massive gas laser or a miniaturized semiconductor laser. You first need a large number of atoms in some kind of medium, whether that’s a solid, liquid, or gas. You then need a stimulant to excite electrons within the medium’s atoms. This stimulant can be something like a flash tube, xenon flash lamp, or even another laser. Lastly, you need a set of mirrors that will bounce photons back and forth, and ultimately out through a hole in one of the mirrors to create our characteristic laser light.
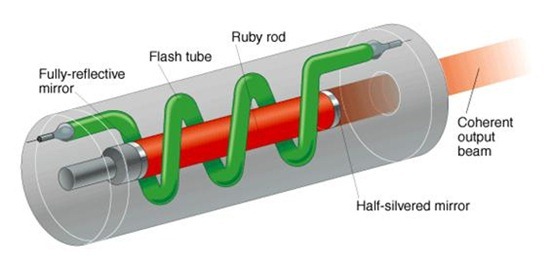
How a laser works
To understand how a laser works, you need to know that electrons sit at various orbits with energy bands within an atom. You can think of these bands as individual steps on a staircase; maybe you have one in your house.
In their default state, all electrons sit on the first step of this staircase, which is considered the electron’s ground state. If you then zap the right amount of energy into an electron, you can get it move up a step. This process is called absorption, where the electron absorbs the energy shot into it, and in the process, its energy level is elevated to the next step or band.
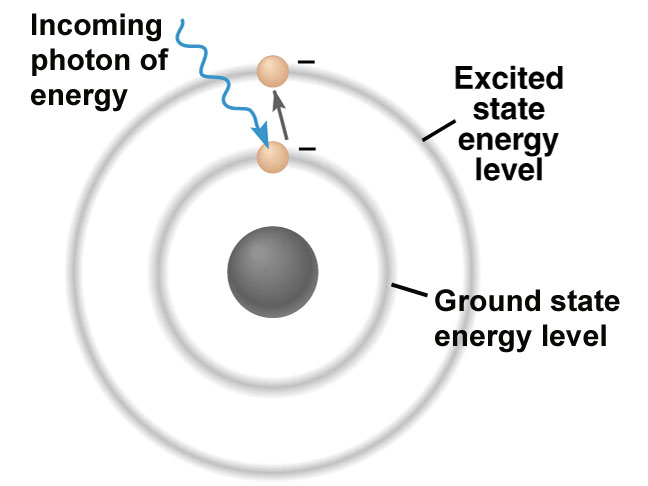
In this higher energy state, the electron is considered to be excited, but also unbalanced. To restore balance, the electron releases the original bit of energy that it absorbed in the form of a photon, or particle of light. This release of energy is called spontaneous emission. Here, the electron loses the energy that is initially gained and steps back down to the first step on our flight of stairs.
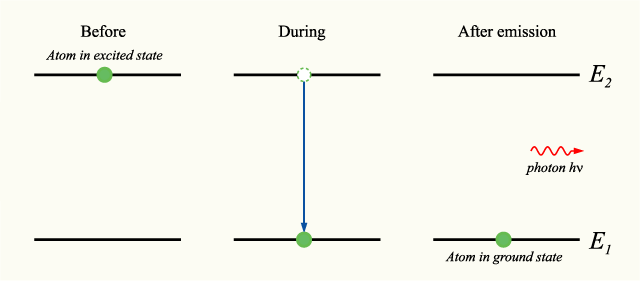
We can see atoms doing this spontaneous emission dance all around us, going from states of ground to excitement and back to ground in a variety of applications. Take for example your toaster oven. The coils burn bright red because atoms are excited by heat, and in the process release red photons. This same process happens in fluorescent lights, computer screens, etc.
Beyond atomic
Now that we understand what’s going on at the atomic level let’s put this together in a practical application of a laser. First, some kind of medium, whether that’s a solid, liquid, or gas, which is exposed to an intense flash of light or electrical discharge. This process creates a massive collection of excited electrons within the medium. When there are more excited electrons than there are grounded electrons in a laser, this state is called population inversion.
All of these excited electrons in their excited state now begin to release the energy that they have absorbed. During this process, an electron will move a few steps down to its original position at the ground while emitting photons of a specific wavelength. These excited electrons are also stimulating other electrons to release their stored photons at the same time. This process of one electron causing a chain reaction of photon release in other electrons is called stimulated emission.

Now imagine that we have a huge amount of electrons alternating from low-to-high-to-low energy states and, in the process, releasing photons. If you now place a set of mirrors between one side of the laser medium and the other you’re able to harness and channel those photons to create our characteristic laser light.
The trick here with the mirrors is that one of the mirrors has to be slightly less reflective than the other. As photons bounce off one mirror, they then hit the slightly transparent mirror, and a small “hole” in the mirror allows a precise beam of light to shine through. Our laser light is born.
You can take something called a ruby laser and see this in action. Check out the image below; this device contains all of the components that we need to make a laser function. It has a medium in the form of a ruby crystal, a flash tube stimulant, and a set of mirrors on either end, one of which is more transparent than the other. Here’s how the process will work here:
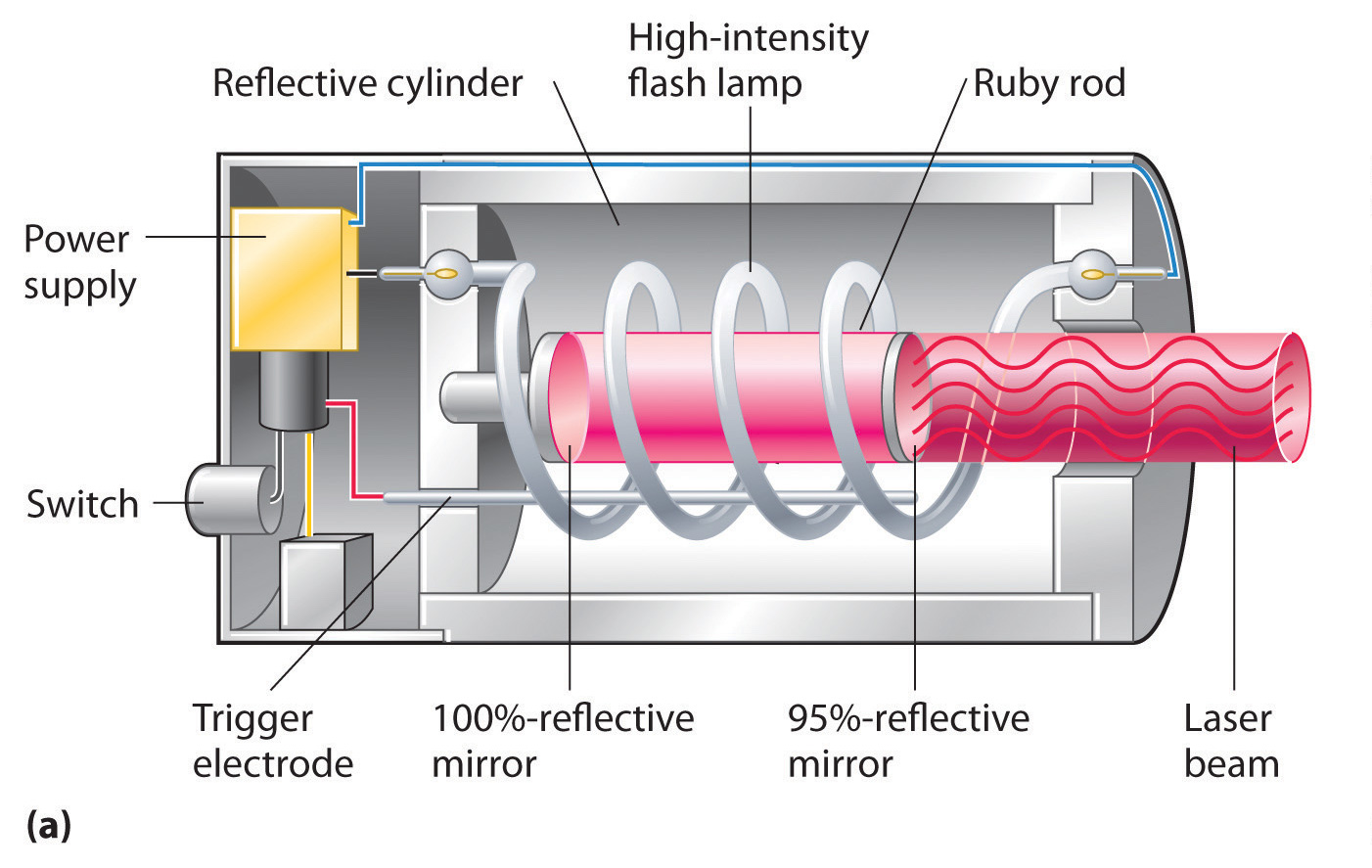
- First, an electric current will make the flash lamp turn on and off, which will excite the electrons in the ruby crystal.
- These excited electrons, in their heightened state, then return to their ground state and give off a photon of light by the process of spontaneous emission.
- These photons zip all around the medium, bouncing off the mirrors and exciting other electrons into heightened states. This causes more photons to be emitted via the process of stimulated emission. Before long you have more excited than grounded electrons, which creates a population inversion.
- The two mirrors keep the photons bouncing back and forth in the crystal medium, but one of the mirrors is slightly less reflective and lets some of the photons through.
- The photons that escape find their way out into the world as a concentrated and powerful beam of laser light.
Types of lasers
There are a variety of lasers, all of which can be categorized based on the type of medium they employ. This can be solid, gas, liquid, or semiconductor. Here’s what to know about each type:
Solid-state lasers
These lasers are made out of a solid medium like ruby or crystalline with a flash tube wrapped around it to excite electrons. Like semiconductors, solid-state laser mediums have to be doped with impurities, which produce light of a specific frequency and wavelength. You’ll typically find these lasers being used for target destination systems in military applications or drilling holes in metals.

Gas lasers
These lasers are typically made out of helium or helium-neon and produce our characteristic red laser light. There are also CO2 lasers which emit energy in infrared. These powerful and efficient lasers are typically used for industrial cutting and welding applications.
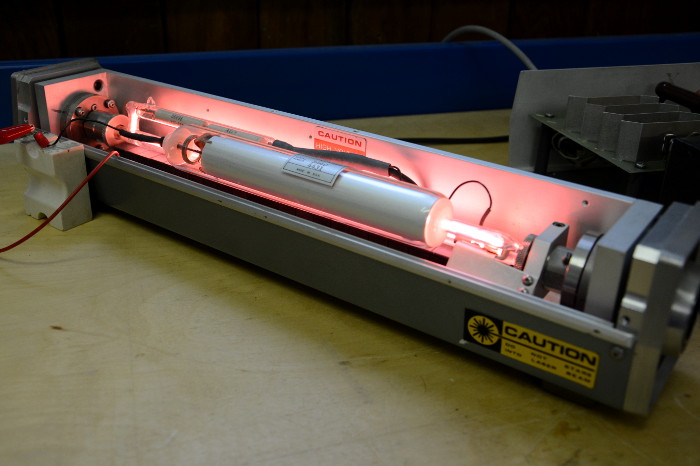
Liquid dye lasers
These lasers use liquid dyes like rhodamine in a liquid solution as their medium. Electrons are excited by either an arc lamp, flash lamp, or another laser. Unlike solid-state or gas lasers, liquid dye lasers can produce a broader band of light frequencies and as a result, can be used in a variety of applications.
Semiconductor lasers
Semiconductor lasers are cheap to produce and are found in various electronic devices from laser printers to barcode scanners. You might hear these lasers referred to as diode lasers, as they take advantage of an LED to generate light in a monochromatic pattern.

Lasers can also be classified beyond their general categories based on specific wavelengths that their medium produces. The most common lasers and their associated wavelengths include:
| Laser Type | Wavelength (nm) |
| Argon fluoride (UV) | 193 |
| Krypton fluoride (UV) | 248 |
| Xenon chloride (UV) | 308 |
| Nitrogen (UV) | 337 |
| Argon (blue) | 488 |
| Argon (green) | 514 |
| Helium-neon (green) | 543 |
| Helium-neon (red) | 633 |
| Rhodamine 6G dye (tunable) | 570-650 |
| Ruby (CrAlO3) (red) | 694 |
| Nd:Yag (NIR) | 1064 |
| Carbon dioxide (FIR) | 10600 |
There is also another system of classification based on the potential for biological damage. You’ll find this class-based system printed on the package of a laser, and it will either be:
- Class I: These are lasers that are not known to cause biological harm. Class I lasers are further broken down into Class I.A, which are not intended to be viewed and include applications like the barcode scanner at your grocery store.
- Class II: These lasers are stronger than Class I but do not have a radiant power above 1 mW. This classification makes them safe for humans to use since our natural aversion to bright light limits exposure.
- Class III: These lasers operate in the 1-5 mW range and are hazardous when the beam is viewed directly. Class III lasers are separated into Class III A, which are intermediate-power lasers, and Class III B, which are moderate-power lasers.
- Class IV: These are high-powered lasers and operate at 500+ mW; they’re also hazardous to view under any condition. When viewed directly Class IV lasers pose a significant skin hazard and can also cause fires if not handled in a controlled facility.
Lasers and their applications
Lasers have a ton of applications that affect our everyday existence. Some are visible, like the use of lasers in tattoo removal, whereas other lasers operate behind the scenes in all of our electronic devices. Some of the most common applications for lasers include:
Cutting & healing
Laser-guided robots are used to cut fabrics and metals that were once cut by hand. Take for example jeans, where laser-guided robots can cut multiple thicknesses of fabric all at once. You’ll also see lasers being used in medicine to destroy cancer tumors, cauterize blood restores, and restore eyesight by repairing detached retinas.
Communicating
Lasers form the foundation of all our connected devices and Internet technologies. The laser-powered barcode scanner at your local store makes buying food easy and efficient. Then there are fiber-optic cables which use photons to transmit enormous streams of data over the Internet.
Defending
Militaries are huge investors in laser technologies and use them for their weapon and missile systems. Back in the 1980s you might have heard of the “Star Wars program” where the US military planned to use x-rays to destroy enemy missiles. Today, the Navy has developed a successful Laser Weapon System (LaWS) for use on their battleships. This weapon system is a solid-state laser that excites electrons with LEDs and can precisely destroy objects at an impressive distance.
Who invented the laser?
That is a contentious question. For starters, we have to give an enormous amount of credit to Albert Einstein who developed the quantum theory of light and photons in 1905. He later went on to theorize the mechanism for stimulated emission in 1917. Without these two discoveries, the development of lasers would have never been possible.
Over 30 years later, we had the first hint of a laser in the form of a maser. This device was invented by American Physicists Charles Townes and Arthur Schawlow. While the maser used the same principles as a laser, it produced microwaves and radio waves instead of visible light. These two inventors went on to earn a Nobel Prize in Physics for their work in 1964 and 1981.

The plot thickens. In 1957, one of Charles Townes’ graduate students, Gordon Gould sketched an idea in his notebook for a visible light version of the maser. Unfortunately for Gould, he never patented his idea and ended up spending the next 20 years of his life fighting for royalties and patents.
So who actually invented the laser? That’s hard to say. Townes and Schawlow are credited for the invention, but the first person to build an actual laser was Theodore Maiman, another American Physicist. However, Maiman’s work was never fully recognized, and his two nominations for the Nobel Prize in Physics went unrecognized.

That’s the abrupt end of the story; we’re sorry to say. There were a lot of hands and minds that went into developing the laser technology that we use today. Some say that the invention was simply a group effort.
Laser away
Just like any other form of electromagnetic radiation, lasers take advantage of visible and invisible light to cut metals, perform eye surgery, scan your groceries, guided missiles, and so much more. What’s amazing is that at the core of this technology are a simple set of principles.
Whether you’re using a gas laser to cut metal or a semiconductor laser in your electronics, each one takes advantage of exciting electrons to produce their required light. With with two simple mirrors, you can channel photons in a concentrated beam to do some amazing work. So look around you, can you spot things in your environment that are powered by lasers? They’re bound to be somewhere.
Want to integrate lasers in your next electronics project? Try Autodesk Fusion for free today!