“Free” Energy: How Solar Panels & Solar Electricity Works to Power Our World
Solar electricity is all around us, from solar-powered pocket calculators to satellites and homes strapped with solar panels. You might even see solar power on your way to work as you pass emergency road signs, call boxes, or speed meters. This “free” source of energy is taking over our modern needs for electricity in a big way and is ultimately paving the path toward a future of sustainable energy. But will it be enough to release our grasp from fossil fuels? Only time will tell. So how exactly do solar electricity and solar panels work? There’s no switch, sometimes not even a battery to charge. We just depend on that giant ball of gas in the sky (the Sun) to power our needs in this world.
Solar Electricity in Action
Solar electricity isn’t actually anything new. In 1839 French scientist Alexandre Edmond Becquerel discovered that a material like metal would send off sparks of electricity when exposed to sunlight. This experiment paved the way for research by other Scientists like Albert Einstein, who later named this process the photoelectric effect.
The photoelectric effect occurs when electrons are ejected from the surface of a solid material like metal when exposed to sunlight. Any material that reacts to this sunlight exposure is considered a photoemissive material, and the electrons that get ejected are called photoelectrons. Don’t let the terms confuse you though; there’s actually nothing different from an electron that gets emitted from exposure to sunlight than one that flies around a circuit from a battery or direct power source. They’re all doing the same job and remain identical in mass, charge, spin, and magnetic movement.

With the photoelectric effect set in stone by Einstein, the world soon turned to creating the first photovoltaic (PV) cells out of selenium. This was just the beginning, and in the 1950s Bell Labs developed a PV cell worthy of meeting today’s solar needs with silicon. This first silicon PV cell achieved a whopping four percentage energy conversion, but at the time the invention was groundbreaking.

Since the 1950s, silicon PV cells have continued to advance in efficiency, but how they function in a solar panel system remains largely the same. Here’s the process at a high-level perspective before we dig into the details further
- Particles of light, called photons, first penetrate a PV cell which transfers their energy to lose electrons. These electrons get knocked off of their orbit in a silicon atom.
- The loose electrons then seek the path of least resistance towards an empty hole in another atom, but like all other methods of manipulating electrons, we first make them do some work by traveling through a circuit.
- As electrons leave a solar cell as electric current, they pass through a wire conduit and into an inverter. This device transforms what is currently stable direct current (DC) into an alternating current (AC) that can provide power for homes, businesses, power plants, and even the electric grid.
- Once our devices and infrastructure are powered, the electric current will then flow back through the circuit of a solar system, finding rest in the solid contact layer at the bottom of a solar panel to create a closed circuit loop.
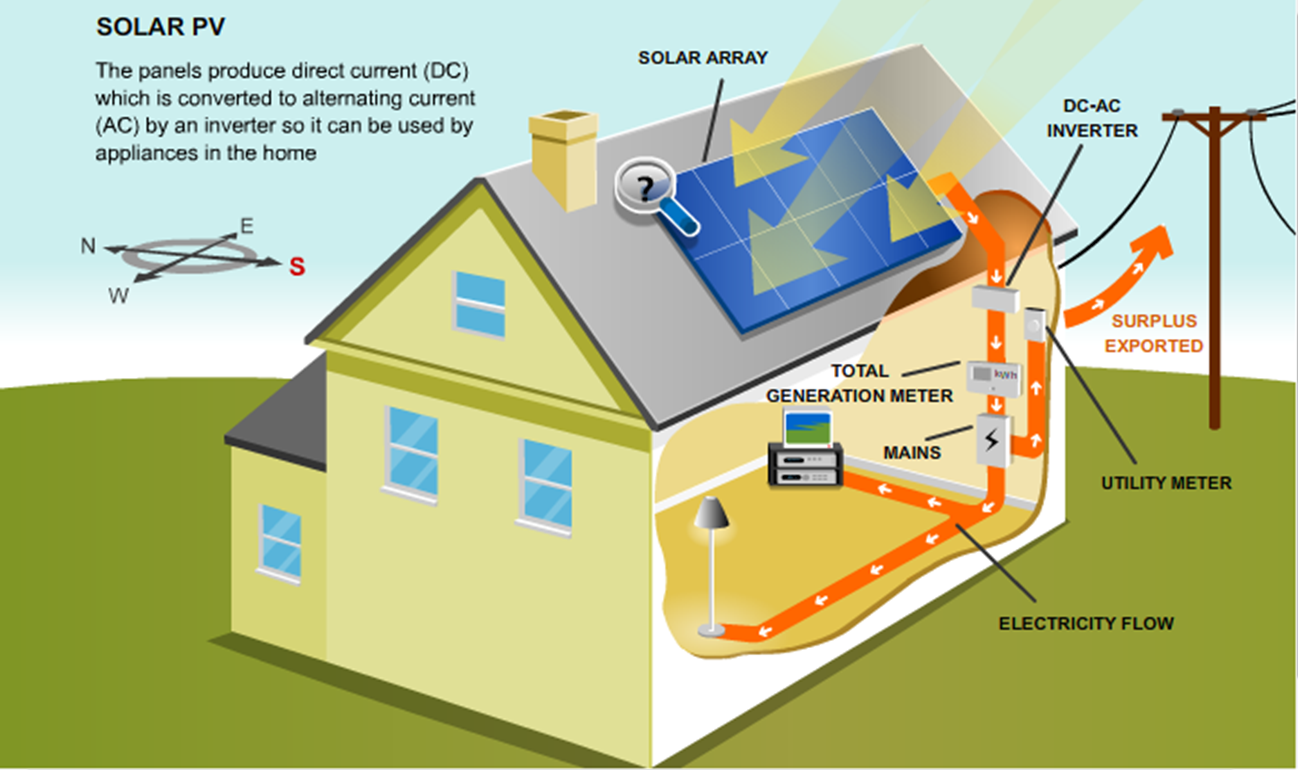
The Inner Workings of Solar Cells
Every solar panel is made up of a collection of individual solar cells, and it’s within these cells where all of the magic happens to convert light energy into solar electricity. Solar cells are typically made up of two inner layers of semiconductor wafers. You might have heard of this material, as they’re currently used in the microprocessor in your computer and the integrated circuits (ICs) that you might use on a printed circuit board (PCB).
Compared with other materials that allow electricity to flow through them easily (conductors), or not at all (inductors), silicon sits somewhere in the middle, neither fully conducting or insulating electricity. Hence the name semiconductor.

In its purest form, silicon generally won’t conduct electricity because 10 of its 14 electrons are already paired. However, the outer shell of silicon only has half (4) as many electrons as it needs, and so it looks to bond with other nearby atoms to gather its remaining electrons and find balance. We’ve figured out a way to take advantage of this electron deficit by encouraging silicon to conduct electricity through the process of doping.
Doping is the process of adding a secondary atom into the crystalline structure of silicon, which changes how many electrons the material has. For example, if you dope silicon with phosphorous, which has five electrons in its outer shell, you now have nine total electrons and one extra electron which can be manipulated to break free and create an electric current. When an electron does break free in doped silicon, it’s considered a free carrier and will be zipping around to search for an empty spot to rest in.
In solar cells, the bottom layer of silicon is doped with boron, which gives the silicon a positive charge, called n-type silicon. The top layer of silicon is doped with phosphorus, which gives this layer of silicon a negative charge, called p-type silicon. And when you put these two layers of n-type and p-type silicon together, you create a junction, called the P-N junction.
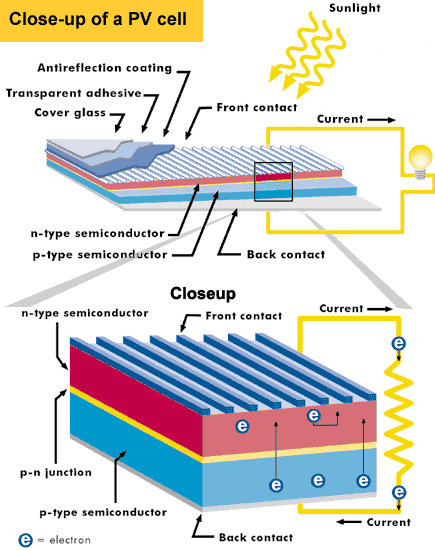
At this point, electrons will be moving about, seeking to resolve their imbalance and in the process creating an electric field. This field acts as a standard diode components, allowing electrons to only flow from the p-type layer to the n-type layer. The problem is, while electrons in the p-type layer can get where they need to go, n-type electrons are blocked by the P-N junction. What are they to do?
By adding an external circuit on top of an n-type layer in a solar cell, you provide a path for your n-type electrons to get to their desired destination in the p-type layer. By following the path of least resistance, the n-type electrons will then flow through a set of thin wires, around a complete circuit to provide power to our homes, and completing the circuit as they collect in the p-type layer. This exchange between n-type and p-type silicon occurs over and over again as light photons knock electrons loose, and so we get a steady flow of current from our solar cells.
Types of Solar Cells
An individual solar cell will only generate a few watts of power, which is why they need to be grouped together to do the heavy lifting. When you group solar cells together to make a larger unit you create a solar module. These modules can then be combined again to form a single solar panel. On the roof of a home, you’ll typically find hundreds of solar cells packed into a set of panels. On larger solar farms you’ll find a ton of solar panels all packed together on massive metal frames, and these form solar arrays to generate a ton of solar electricity.
Whether it’s a solar panel or a solar array, solar cells don’t just exist in isolation. Silicon is a highly reflective material, and if we expose this material to sunlight, all of the light will bounce off of it. To solve this problem, manufacturers typically apply an anti-reflective coating to the silicon to minimize any loss when capturing light energy. This coated silicon will then be covered with a glass cover plate, which gives a solar cell its typical blue/black opaque color.
Regardless of how many solar cells you might be packing into a panel, they can all be broken down into one of three types:
Single-crystal cells
These solar cells are first made form long cylinders of silicon and are then sliced into thin wafers. The manufacturing process to make single-crystal cells is very precise and produces cells that have the highest efficiency rating of all the types at 23%.
Polycrystalline cells
These solar cells are first made out of molten silicon casts, which then get sliced into thin squares. The manufacturing process for polycrystalline is inexpensive compared to single crystals but also results in a lower energy conversion efficiency of around 20%.
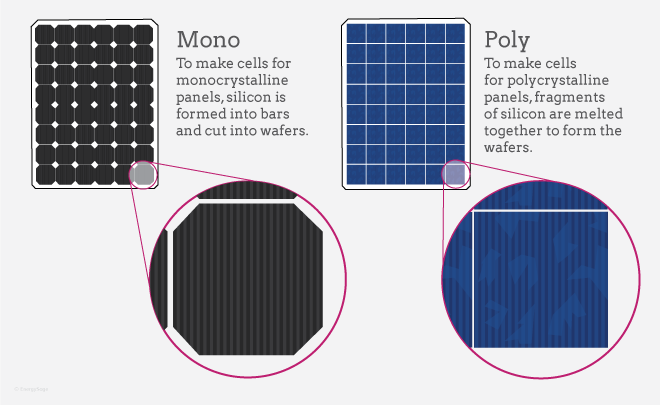
Thin-film solar cells
These solar cells are nearly 100 times thinner than single-crystal or polycrystalline cells, and you might find them made from alternative materials like cadmium-telluride (CdTe) or copper indium gallium diselenide (CIGS). Due to their lightness and flexibility, these “second generation” cells can be applied to a variety of backing materials like metal, plastic, or glass. However, this boost in flexibility also leads to a drop in efficiency of around 7-12%.

Efficiency Problems
At this point you might be wondering – why in the world are we producing solar cells that are only capturing at most 20% of available sunlight? Even theoretical maximums for solar cell efficiency limit them to 30% according to the Shockley-Queisser limit. What’s the problem here?
Sunlight doesn’t just produce one kind of photon. If you’ve ever seen the electromagnetic spectrum, then you know that light actually comes in many varieties, many of which aren’t visible. All of these spectrums of light are based on a specific frequency and wavelength.
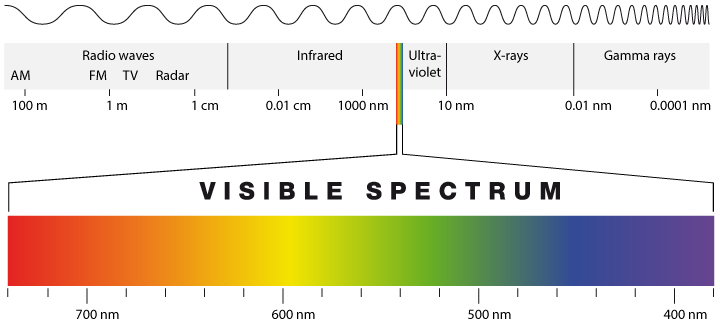
Silicon is only optimized to capture a particular frequency band of photons. For example, some photons that hit a silicon-based cell don’t have enough energy to knock electrons loose. Other photons might have too much energy and will knock an electron loose, but any excess energy is wasted in the process. There is only a precise amount of photon energy, measured in electron volts (eV) that is required to knock an electron loose. For silicon, about 1.1 eV will get the job done. Any photon energy above and below this threshold ultimately winds up as wasted potential.
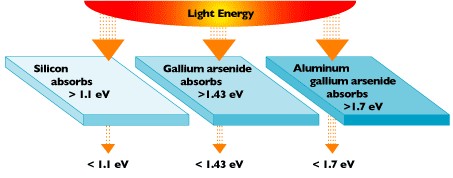
The frequency band of photons isn’t the only problem impacting solar efficiency. There’s also the issue of how far the electrons have to travel within a solar panel until they reach their intended destination. Because silicon is a semiconductor, any electron that travels along it will encounter a fairly high resistance. The further away you place your terminal contacts from the silicon material on a panel, the farther your electrons have to travel, and thus more resistance. As any informed electronics designer knows, the higher the resistance, the more energy you lose.
To minimize this loss, solar cells are typically covered with a metal grid that shortens the distance they have to travel to a terminal connection. Some manufacturers also stack solar cells made from a variety of materials that each have a different energy band gap. This multi-material stackup allows more frequencies of photons to be absorbed, which increases the overall efficiency of the solar cell.
Weighing the Pros and Cons of Solar
Is solar technology worth it? When relied on as the sole means of power production, solar still presents many challenges. However, when used in combination with other sources of electricity, you’ll find solar at its best.
The Pros
Take for example the ability to solar panels to generate electricity on an as-needed basis in its immediate vicinity. A home equipped with solar panels on its roof will be producing electricity right where it’s required. This can help utility companies to avoid excessive demand and strain on their distribution and transmission systems by allowing homes or businesses to source their power needs on-site via solar panels.
On a hot summer day when everyone is running their air conditioning at full blast, solar panels provide the perfect energy balance. You have your immediate energy needs met by the solar panels, which reduces strain on transmission systems from the grid. All of this leads to fewer blackouts, and ultimately a system less dependent on a single point of failure for powering the world.
There’s also the benefit of solar being a modular technology. In a situation where a single solar panel is damaged, the rest of the system can continue working. This is in contrast to something like a nuclear power plant, which is prone to complete failure if one system stops working.
The Cons
There are also some challenges for solar technology. For example, when a home doesn’t consume all of the power it generates from solar this energy is typically fed back into the grid through “feeder” lines. Implementing these lines can be a costly expense for utility companies as solar use expands in the future.
There’s also the issue of how solar is transmitted to its users. While solar panels on a home provide energy where it’s needed, there are also massive solar farms that have to transmit all of their generated electricity via transmission lines. Like any material that current travels on, there’s always a loss in energy during the transmission process in the form of heat that can never be regained.
The last major challenge is simply the fact that sunlight is not a constant variable that can be triggered on and off at our choosing. When it’s cloudy, energy generation from solar panels plummets. Because of this, many utility companies use solar in combination with other energy sources to balance out daily demands.
The Future of Solar Technology
Walk around any neighborhood in the United States, and you’re bound to see more solar panels being put to use year after year. In the last ten years alone the price of solar technology has dropped more than 60%, making this once expensive technology available average households and businesses.
But what about advancements in solar efficiency? There’s a growing amount of research and development underway to create more efficient solar technologies. One of these is the perovskite solar cell. In its current phase, the perovskite cell is proving to be cheaper than silicon cells while being just as efficient. This material is made out of a calcium titanium oxide crystalline structure and can be manufactured at room temperature with much simpler methods than silicon requires.
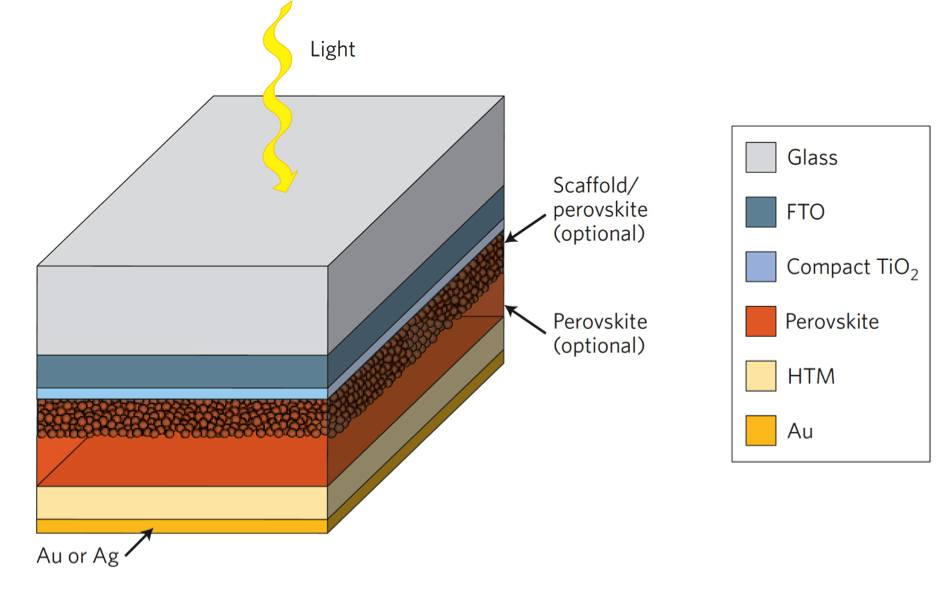
Will this material replace silicon cells completely? Not likely. Companies are instead looking into ways to combine perovskite and silicon in the same cell. By doing this, both materials will be able to capture photons at different wavelengths, which adds to the overall efficiency of a cell.
Researchers say we’re still a good 5-10 years off from seeing perovskite ready for prime time, but early experiments look promising with 20% efficiency, which is in line with silicon. But will perovskite ever be able to dethrone silicon as the industry leader? It’s going to be an uphill battle if that’s the goal.
Can Solar Save the World?
Can we rely on solar energy alone to power the world with a sustainable source of energy? There are a ton of variables that need improvements, like efficiency enhancements and the continued development of infrastructure. We think the future might present a reality where solar is just one small piece of a larger sustainable energy puzzle.
It’s fascinating to learn how even solar cells rely on the basic foundations of electricity to make their magic happen. It all starts with silicon semiconductors which interact with photons from sunlight to generate a usable electric current. From there, we can use the basic principles of circuits as we do in all of our electronic devices to power our homes, businesses, and ways of life.
Got a great idea for a solar powered electronics project? Subscribe to Autodesk EAGLE today!