Explore the three laws of thermodynamics: energy conservation, the law of entropy, and absolute-zero behavior. Plus real-life examples that make these thermodynamics laws easy to grasp.
Everything is thermodynamics. This isn’t just a concept reserved for the realm of physics, and it’s not a set of laws that you’ll only find in electronics, refrigerators, cars, planes, etc. This is a scientific concept that weaves itself into the very fabric of life.
What are the Laws of Thermodynamics?
The laws of thermodynamics describe how energy, heat, and entropy behave in physical systems. The zeroth law defines thermal equilibrium. The first law states energy cannot be created or destroyed, only transformed. The second law says entropy increases over time. The third law states entropy approaches zero as temperature nears absolute zero.
Let’s take a deep dive into each below.
The Three Laws of Thermodynamics: Energy, Entropy & Equilibrium
The problem is, that thermodynamics is just the way energy works, so it’s easy to miss. When you clean your office week after week, which seems to get messier by the day, that’s the Second Law of Thermodynamics at work. Everything leads to an increased state of disorganization.
Or when you cook that delicious steak on the grill this weekend, that’s the First Law of Thermodynamics at work. Energy is transferred in the form of heat to your food. Thermodynamics is not just the study of heat and work. It’s the study of how energy, the stuff that you and I and everything around you are made of, works. Thermodynamics is the study of life.
Systems and surroundings
We all live in a system where matter and energy are being continuously exchanged; it’s an endless flow. Take, for example, the process of eating. You take in the chemical energy of food and convert it into a form that can be used by your body. Now that your body has energy gained from food, it can go about doing work out in the world.
This process of exchange, where energy transforms from one state to another all happens within a set of systems and surroundings. When you turn on your electric tea kettle in the morning, you have water enclosed within a metal container; that’s your system. The rest of the kitchen and even the rest of the house are the surroundings.
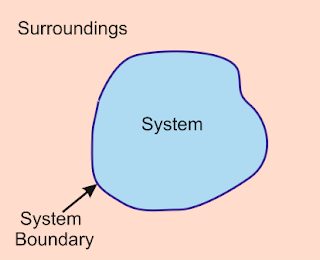
When your tea kettle starts to boil, it transforms some of the water into steam which releases from the spout at the top. This converted energy crosses a boundary from the system within the metal container to the surroundings outside of it. This is thermodynamics at work—the transference of energy and matter between systems and surroundings.
What are systems and surroundings?
Systems are defined by the observer. So, to one person, the tea kettle might be the system. To another, the entire house might be the system, and the neighborhood the surroundings. It all depends on your perspective. The point is that every system in thermodynamics is contained within a defined boundary, and on the other side of the boundary are the surroundings. There are three types of systems in thermodynamics:
- An open system which is where energy and matter can be exchanged between a system and its surroundings.
- A closed system is where only energy can be exchanged between a system, and its surroundings don’t matter.
- An isolated system is where no energy or matter is exchanged between a system and its surroundings. A truly isolated system is rare.
At a high level, our entire universe is considered a system. But what are the boundaries of our universe, and what are its surroundings? Those are some of the bigger questions we have yet to answer.
For the electronics designer, thermodynamics presents a more personal reality with the everyday devices you design. You’ll find that many of the principles you already work with to calculate and analyze circuits, like Kirchhoff’s Law, are based on the foundations of thermodynamics.
Learn about the Three Laws of Thermodynamics (including the law of conservation of energy and the law of entropy) and see how they govern everything from engines to everyday life.
The First Law of Thermodynamics
The First Law of Thermodynamics, also known as the Law of the Conservation of Energy, says that energy cannot be created or destroyed; it can only change form. Energy comes in a ton of different forms, including:
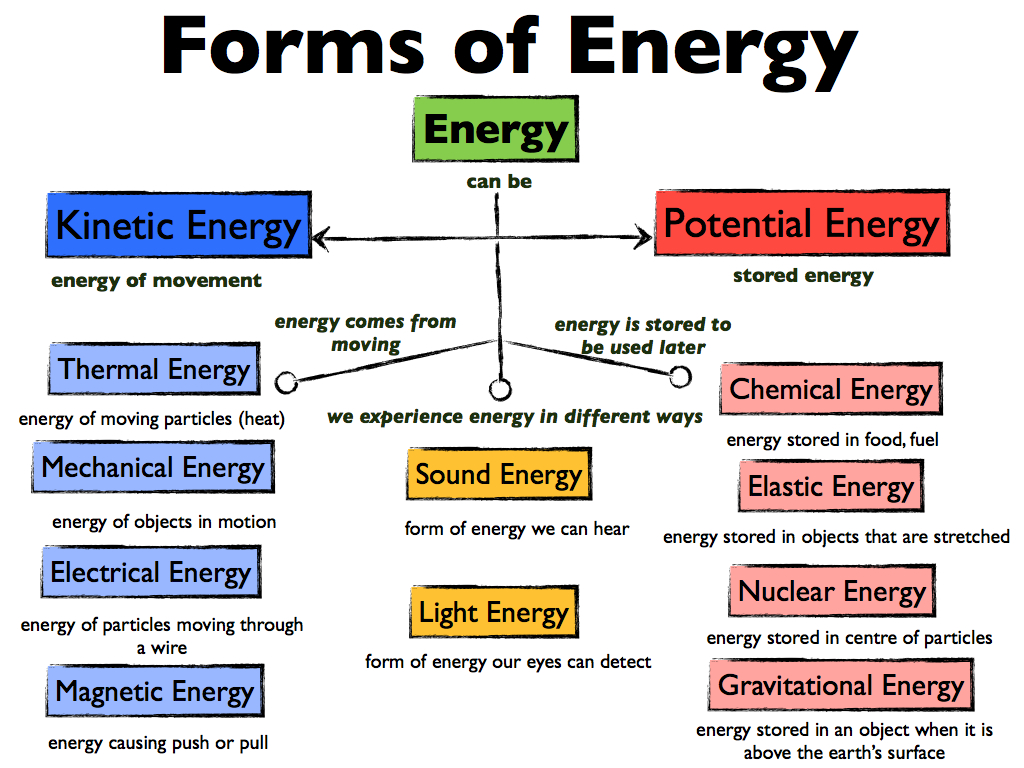
Energy is not created or destroyed; it simply changes from one form to another. Turning on a light switch does not create energy, it simply converts electrical energy into radiant energy (light) and thermal energy (heat).
Within the First Law are three related concepts – work, heat, and internal energy. Heat is the transfer of thermal energy between two systems. Work is the force that transfers energy between a system and its surroundings. By producing work either within a system or outside of it, you create heat. Then there’s internal energy, which is all the energy within a system. When heat, work, and internal energy interact together, energy is transformed.
You can express this relationship mathematically as:

Here, ΔU is the total change in internal energy within a system, Q is the heat exchanged between the system and its surroundings, and W is the work done to or by the system.
Examples
When a system releases heat or does some kind of work, the internal energy of the system decreases. Likewise, if heat is added to a system or if work is done to a system, the internal energy of the system will increase. Any kind of energy that is released by a system is absorbed by its surroundings, and any kind of energy lost by a surrounding is absorbed into a system. In all of these examples, you aren’t creating or destroying energy; it’s just moving from one place to another.
One important thing to keep in mind about the First Law is that the transformation of energy is not 100% efficient. In our light bulb example, you can transform electrical energy into a usable form of light energy. However, in the process, you create unusable energy in the form of heat.
When related to electronics, the First Law of Thermodynamics resembles Kirchhoff’s Current Law. This well-known law states that the amount of current that enters a node is equal to the amount of current leaving a node. It doesn’t matter how many nodes you have, what goes in, must come out.
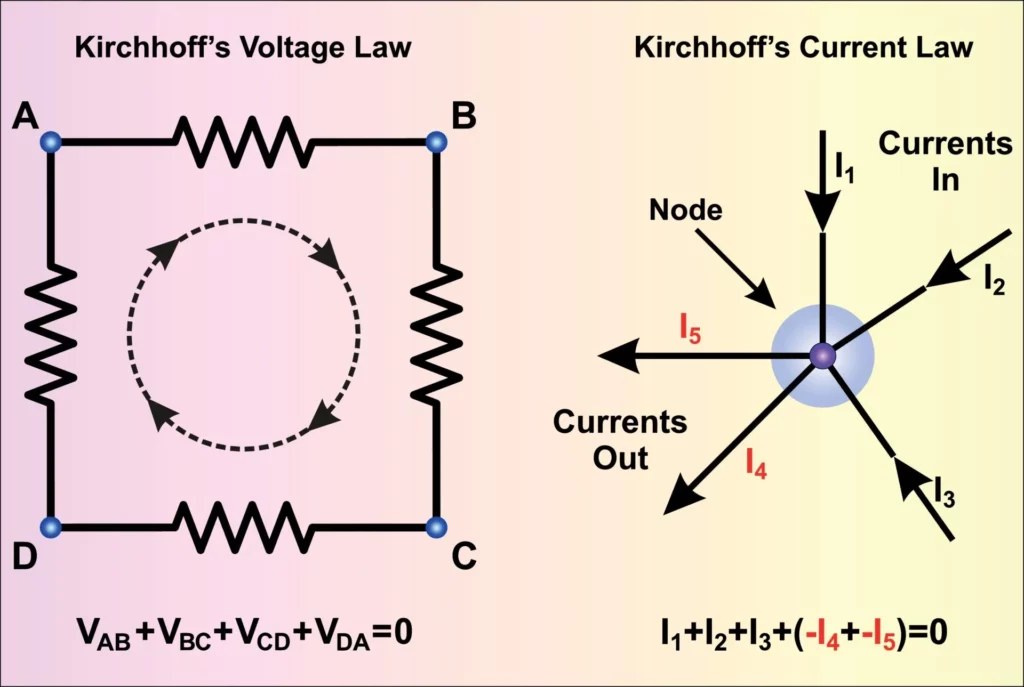
Doesn’t that look strangely familiar to our equation for the balance between systems and surroundings?
The Second Law of Thermodynamics
The Second Law of Thermodynamics, also known as the Law of Increased Entropy (or simply the law of entropy), says that over time the state of disorganization or entropy in a system will always increase. What do we mean by this? Take this example: why does your desk always get messier as the week progresses? Or, more importantly, why doesn’t your office go from messy to clean without you having to do work on it? This is the arrow of time in thermodynamics. As time increases, so too does disorganization.
This phenomenon happens in any system. Over time, usable energy will eventually give way to unusable energy. While energy cannot be created or destroyed according to the First Law, it can change from a useful state to a less-useful state, like thermal energy (heat).
In our light bulb example again, the longer we leave our light bulb on, converting electrical energy into radiant energy, the more usable energy we continue to convert into unusable energy in the form of heat. As usable energy within a system, decreases and unusable energy increases, then we say that the entropy of a system has increased. Stated mathematically:

Here, the total entropy ΔSuniverse within the universe equals the total entropy within a system ΔSsys plus the total energy within all surroundings ΔSsurr, all of which cannot be less than 0. Why? Because at all times, at all hours of the day, all energy is being transformed from one form into another, and one of those forms is unusable energy. Driving in your car uses mechanical energy to produce the kinetic energy of motion, but in the process, you also transform a ton of energy into heat. It’s an inevitable byproduct.
Examples
Another way to think about entropy is with probabilities. Take a box filled with puzzle pieces as an example. What’s the probability that you dump all of the puzzle pieces out of the box, and one of the pieces randomly lands where it connects perfectly with another piece? It’s a low probability. In that same box, what’s the probability of a piece landing randomly where it doesn’t fit with another piece? It’s a high probability.

In this puzzle example, the randomly placed puzzle piece represents a higher form of disorder or entropy. This is why tires release air when punctured, or why ice cubs left out at room temperature eventually melts, or why the electrons in a circuit flow from negative to positive. Sure, it could be possible for all of these actions to occur in reverse, but the probability of them occurring is so low, and the cards of increasing probability are stacked so high, that they simply never occur.
In electronics, we see the Second Law of Thermodynamics at work with the Seebeck Effect. This phenomenon occurs when heat is applied to one of two conductors, which causes heated electrons to flow toward the cooler conductor. If you connect this pair of heated conductors together in a circuit, then the heating effect will cause a direct current (DC) to flow through the circuit. In this situation, we have electrons in a lower state of entropy in a cold conductor reaching a higher state of entropy through the application of heat, and so disorder increases.

The Third Law of Thermodynamics
The Third Law of Thermodynamics says that a perfect crystalline structure at absolute zero temperatures will have zero disorder or entropy. However, if there is even the smallest hint of imperfection in this crystalline structure, then there will also be a minimal amount of entropy. This law gets a little strange, though, because even at zero Kelvin there is still some atomic movement happening, so it’s a bit theoretical. Regardless, this law allows us to understand that as the entropy of a system approaches a temperature of absolute zero, the entropy present within a system decreases.
The Zeroth Law of Thermodynamics
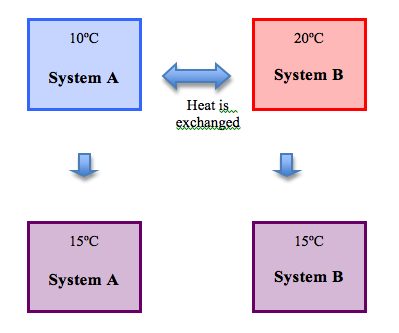
The Zeroth Law of Thermodynamics says that if two systems are in thermal equilibrium with a third system, then the first two systems are also in thermal equilibrium with one another. Using our good old Transitive Property of Equality:
- If System A is in balance with System C
- And System B is in balance with System C
- Then System A and System B are also in balance with each other.
This law allows you to define the direction of heat flow between systems. If you know the temperature of a set of connected systems, then you’ll know which direction heat will travel based on the fundamentals of thermal equilibrium.
Note that while we’re covering the Zeroth Law last, it actually comes first. In the 18th century, when the Laws of Thermodynamics were defined, only the first three were included. However, scientists realized that they needed a fourth law that defined the movement of temperature. Rather than renumber all of the existing laws and add confusion to existing literature, English scientist Robert Fowler came up with the name Zeroth Law.
Who Discovered The Laws of Thermodynamics?
The Laws of Thermodynamics were not discovered by one person. The development dates back as far as the 1600s when the basic idea of heat and temperature were first being formulated. In 1824, French physicist Sadi Carnot was the first to define the basic principles of thermodynamics in his discussions on the efficiency of an ideal machine. Sadi originally used the caloric system for describing the heat that is lost during the motion of an engine, which was later replaced with entropy in the Second Law of Thermodynamics.

In 1850, German physicist Rudolf Clausius developed the Clausius statement, which said that “Heat generally cannot flow spontaneously from a material at a lower temperature to a material at a higher temperature.” Around the same time, William Thomson (Lord Kelvin), developed the Kelvin statement, which said that “It is impossible to convert heat completely in a cyclic process [without losing energy].” Both of these statements went on to form the foundation of the First and Second Laws of Thermodynamics. The Third Law of Thermodynamics was later developed by German Chemist Walther Nernst and is often referred to as Nernst’s Theorem.

Laws of thermodynamics in action
Look all around you at this amazing world of energy in motion, and you’ll see the Laws of Thermodynamics in action. Whether it’s in the process of converting the chemical energy of food into usable energy in your body or converting mechanical energy into kinetic energy in a car or airplane.
Thermodynamics is a way of life. You’ll even find the Laws of Thermodynamics at work in your electronic designs. Principles like Kirchoff’s Current Law line up perfectly with the First Law of Thermodynamics, stating that the current that goes into a set of nodes must come out, just like how energy cannot be created or destroyed, only transformed.
For the Second Law of Thermodynamics, we have the Seebeck Effect to observe in electrical circuits. Heated electronics will flow towards a cooler conductor and, in the process, create the flow of current in a circuit. Here we have entropy in action, creating increasing states of disorder wherever it goes.
Whatever you might be designing, you’re designing with the Laws of Thermodynamics. Put these principles into action with Autodesk Fusion electronics today.